Updated Every Month
As COVID-19 social distancing interventions continue to be relaxed in many countries and the world approaches a possible endemic stage of the pandemic, vaccination continues to be pushed as an effective strategy as new variants of COVID-19 continue to emerge.
Simultaneously, progress is also being made around new treatment methods for those who do get COVID-19, to avoid hospitalization and speed recovery – so called “therapeutics”. Our infographics have continually demonstrated the continued efforts of African governments to secure vaccines for their populations, despite hoarding elsewhere, high prices and constraints on local manufacturing. But are new COVID-19 therapeutics any different? Are these better available to African countries? This question is what this month’s infographic tries to answer.
First, a brief explanation of the therapeutics. These are medicines an infected person could use, ideally administered not only in hospital but from the comfort of their home with a prescription, or as an injection or other delivery method offered at a health centre.
Most of the medicines that could be used this way are still being assessed for efficacy and safety and are strictly for use in mild-moderate cases where hospitalization is a risk. However, two treatments of note have now passed the World Health Organisation Emergency Use Authorization (EUA) muster: the Pfizer brand PAXLOVID and Merck-Ridgeback Biotherapeutics LAGVERIO. Both can be administered as oral tablets, making them convenient for prescription use at home by individuals, with efficacy rates of 89% and 30% respectively.
Producing these drugs requires licensing agreements, because the firms that produce them -Pfizer and Merck-Ridgeback – have sought Intellectual Property (IP) protection through international patents, introduced through the World Trade Organisation (WTO) under an agreement known as Trade-Related Intellectual Property Rights (TRIPS) (as an aside – this history of TRIPS is essential reading for both the un-inititiated and initiated!). This makes these original versions of drugs very expensive – over 500 USD for just one course of treatment for both types. Of course, this kind of cost is beyond the reach of most low- and middle-income countries, including in Africa.
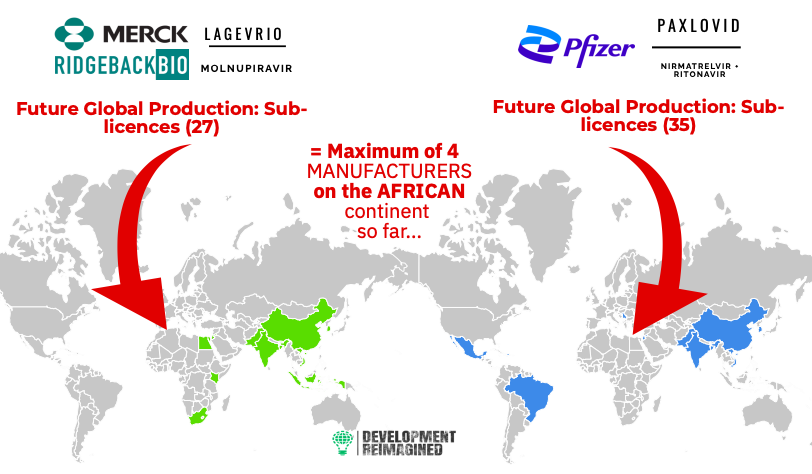
As such, somewhat cheaper sub-licenses of both drugs have been facilitated by an international arrangement known as the “Medicines Patent Pool”, with 35 sublicenses for PAXLOVID in 12 countries, and 27 sub-licences in 11 countries for LAGVERIO. The medicines that could be produced under this arrangement are called “generics”. Their cost is significantly lower – as low as 20 or 25 USD in some cases.
However, so far (by June 2022), there are no African manufacturers licensed to manufacture PAXLOVID, but there are at least 4 for LAGVERIO. It can therefore be inferred that this will make PAXLOVID both harder to procure and more expensive in Africa than LAGVERIO.
Further to that, PAXLOVID’s key compound nirmatrelvir takes 7 to 9 months for Pfizer to produce, hence available supplies of the drug are limited and have already been secured by richer countries, a trend we are familiar with for vaccines. As global generic supply is not estimated to pick up until 2023, most supply of these medicines will have to come from Pfizer and Merck. This introduces similar supply constraints as were seen during the vaccine supply bottlenecks of the COVID-19 pandemic lockdown period.
So, where are these two medicines and their ingredients available currently?
The answer: mostly in the United States. Of the 29 million currently available PAXLOVID courses, the US alone has secured 20 million of these – representing nearly 70% of available PAXLOVID treatments; of the 12 million available LAGVERIO doses, the US holds 3.1 million (25%). In comparison, no African country has managed to secure any purchase agreements for PAXLOVID, but at least one African country has a purchase agreement for LAGVERIO – Egypt. It bears mentioning that the 20,000 courses of LAGVERIO Egypt secured barely counts for 0.17% of the available LAGVERIO stock of 12 million courses. However, UNICEF has managed to secure 3 million courses (25%) of LAGVERIO stock, which could be dedicated for use in emergency cases across low-and-middle-income countries, but even these amounts would not be enough to meet all African countries’ needs for COVID-19 therapies.
For African countries, inequities in new therapeutics availability create a dangerous context, with global public health crowd restrictions measures waning, new COVID-19 variants are expected to emerge, and infection/ re-infection outcomes changing unpredictably. As our infographic shows, still today less than 20% of the African population is fully vaccinated, with only 17 (often the relatively richer) countries of the 55 on the continent having vaccinated over a quarter of their populations.
So what next? Our data suggests two key action points:
First, the world must move in the direction of enabling local production by enabling more sub-licencing and generics production, to permit the rapid sharing and proliferation of treatment options for the most vulnerable countries and populations. However, African publics saw a disappointing end to the June 2022 WTO negotiations on a proposal to waive IP rights. In the end, the decision settled on offering limited waivers for vaccines, but excluded trade secrets and manufacturing methods, and deferred waivers for therapeutics and other essential items for another 6 months. Yet, these are literally life and death issues. How many lives will be lost in this 6-month period, or longer?
Second, albeit highly dependent on the above, Africa must grow its vaccine and therapeutics manufacturing capacity, including beyond fill-and-finish facilities to include API production – and build the associated expertise necessary. Not only is there significant economic incentive now for this on the continent, but it will also avoid the effects of richer countries hoarding resources, while improving future access to innovation, such as new treatments and diagnostics for the health challenges of the future.
We’ve set out and explained our data with several enlightening visuals below – have a read, check out the graphics and numbers – do let us know what questions these have raised with you, and what you’d like us to find out next time!

To find out how Development Reimagined can support you, your organisation or government to respond to COVID-19 and other global crises in a sustainable way, please email the team at clients@developmentreimagined.com.
Special thanks go to Osaru Omosigho, David Tinashe Nyagweta, Sena Voncujovi, Chenyu Wang and Qiu Yu for their work on the graphics and collecting/analysing the underlying data and preparing this accompanying article.
The data was collated primarily from Africa CDC, as well as other sources including: government websites and media reports, the IMF policy tracker; Worldometer and the New York Times Vaccine Tracker. Our methodology is entirely in-house, based on analysis of vaccines, testing, spending, social distancing, income categories and other trends.
If you spot any gaps or have any enquiries, please send your feedback to us at interns@developmentreimagined.com , we will aim to respond ASAP!
July 2022