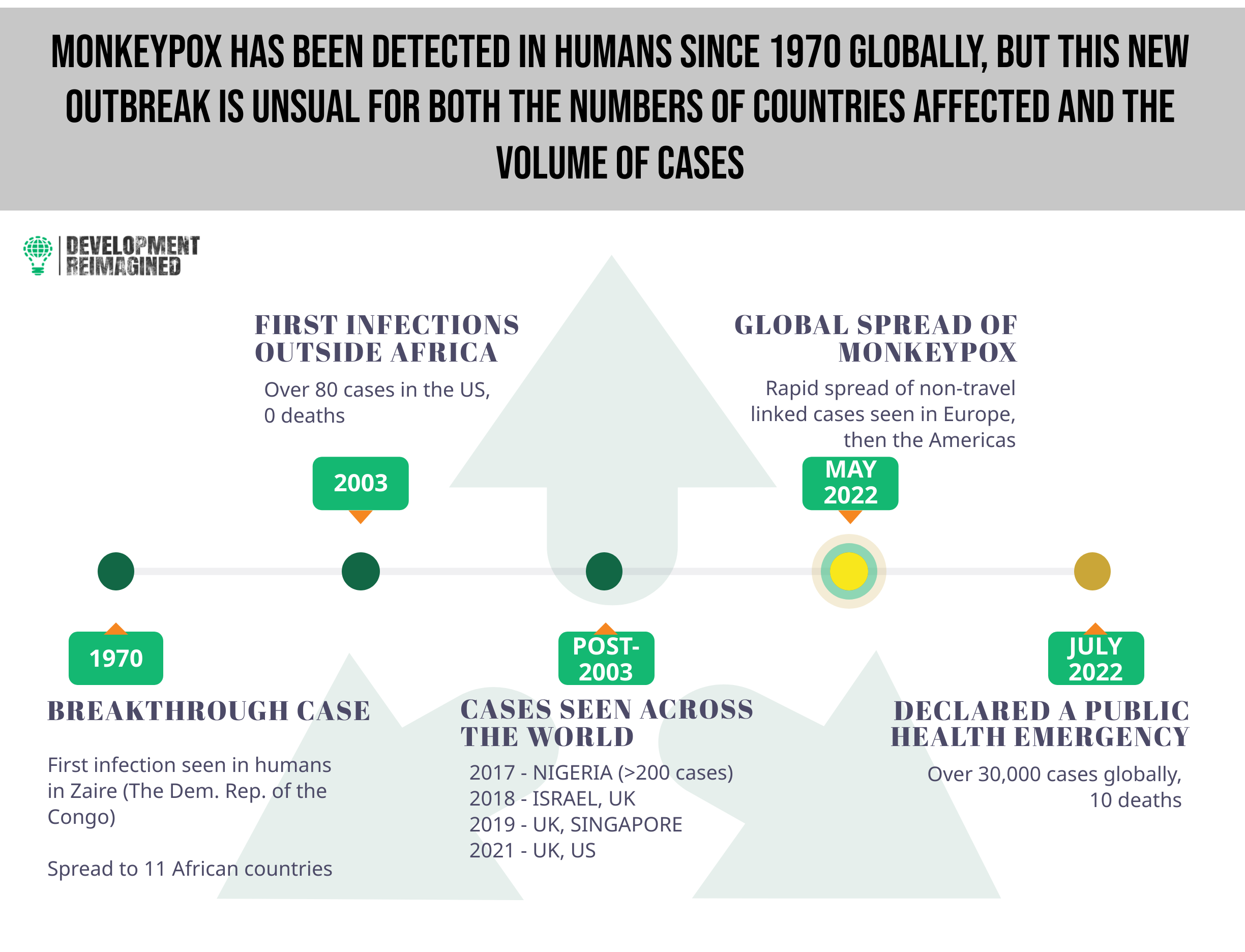
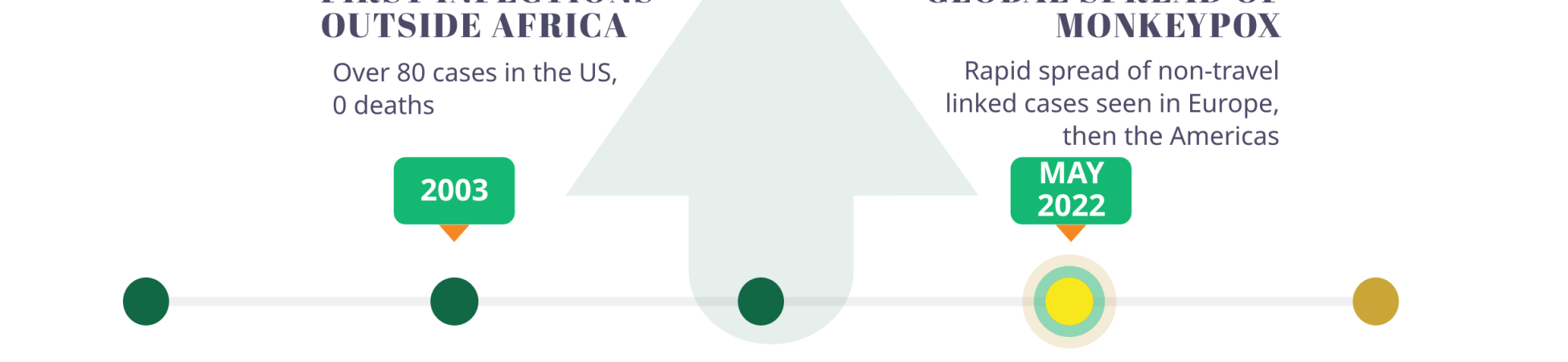
Named for its first detection in 1958 in lab primates, monkeypox had previously been considered as a pathogen of high priority by the WHO. Perhaps due to its low fatality rate and/or its typical detection in a few West African countries – barring an outbreak in the US in 2003 – the disease received little global attention until the current outbreak. For instance, in 2017, Nigeria experienced an outbreak of over 200 confirmed cases (~500 suspected cases), with a case fatality rate of 3%.
The WHO had previously classed monkeypox viral infections into two clades – Clade I and II (formerly known as the Central African clade and the West African clade respectively), and the Africa CDC (as at August 11, 2022) classed the disease as a moderate risk, given that it is not easily transmissible, is self-limiting within 2 – 4 weeks with low mortality, and lacks effective treatment.
In May 2022, the strain Clade IIb was detected in the United Kingdom and has since been detected in almost 90 countries, registering over 40,000 confirmed cases and 15 deaths globally, prompting a scramble for vaccines as a preventive measure.
As an orthopox virus, monkeypox can be inoculated against with therapeutics intended for smallpox. However, due to the eradication of smallpox in 1980, orthopox vaccine stocks and production are fairly low, and have not been ramped up previously for monkeypox outbreaks in African countries.
Current orthopox vaccines are limited to: Bavarian Nordic’s injectable Imvanex (also known as Jynneos) – which is globally approved by the WHO and has at least 85% efficacy; Emergent Biosolutions’ older and side-effect-prone treatment ACAM2000 and its recently acquired Tembexa suspension, and SIGA Technologies’ oral Tpoxx, which was licensed in 2022, but is still in assessment. A further option is Japan’s KM Biologics LC16, which is licensed by the WHO for use in children.
Based on the new outbreak, the WHO has estimated current global demand for orthopox vaccines at around 10 million doses. These are most crucial to deploy both where the disease is spreading and where death is more likely. That means the vaccines should in principle be available in African countries, as well as high income countries.
The reality is that 16 million doses of Imvanex are currently available. However, 15 million of these are still in bulk formulation and have reportedly been promised to a limited group of high-income countries such as the US, Canada, and the EU, with the remaining approximately 1 million finished doses held in stockpile by the US.
Furthermore, Bavarian Nordic – the only producer of Imvanex – is only capable of producing 30 million doses per year, and have closed their facility until late 2022 due to other vaccine manufacturing. Biologics has not expressed interest in production scaling or distribution from its stockpiles, the amounts of which remain unknown.
Will these companies sign more licensing agreements with manufacturers in developing countries to improve availability of the drug globally – such as with South Africa’s Aspen Pharmacare, which has expressed interest in taking up fill-and-finish for the vaccines locally? This would at least enable some production of smallpox antivirals in Africa…
Without this, right now, the continent risks repetition of the COVID-19 testing, PPEs, vaccines, and therapeutics supply delays, highlighting the need for international rules on global health to adapt with the times: vaccine research, development and supply of treatments must prioritise manufacturing and availability in low-income, vulnerable regions – particularly those in Africa – for humanity to get ahead of the next big outbreak.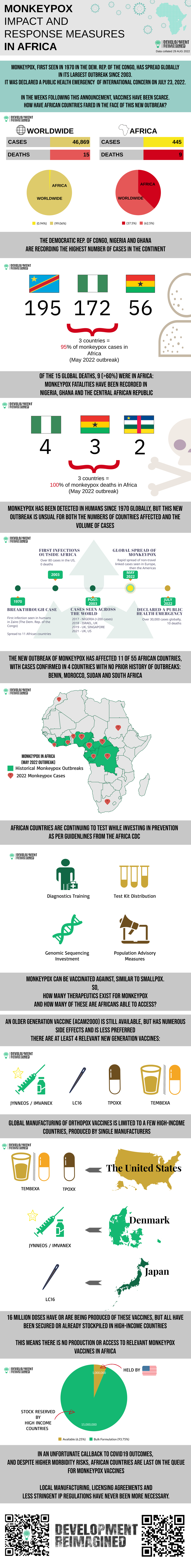 Our data was collated and analysed from several sources, such as the Africa CDC outbreak updates, the WHO Global Trends (including its Health Emergency Dashboard), as well as multiple government and media releases.
Our data was collated and analysed from several sources, such as the Africa CDC outbreak updates, the WHO Global Trends (including its Health Emergency Dashboard), as well as multiple government and media releases.
Special thanks go to Osaru Omosigho, David Tinashe Nyagweta, Rugare Mukanganga and Sena Voncujovi for their work on the graphics, collating/analysing the underlying data and the preparation of this accompanying article.
If you spot any gaps or have any enquiries, please send your feedback to us at interns@developmentreimagined.com, we will aim to respond ASAP!
August 2022